In November 2019, the latest research result "Dissecting primate early post-implantation development using long-term in vitro embryo culture" was completed by the Institute of Translational Medicine of Kunming University of Science and Technology and the Shenzhen BGI Research Published online in the internationally renowned academic journal Science.
The article uses single-cell sequencing technology to analyze the important molecular and cellular biological events of primate embryos after implantation, especially during the intestinal movement, which has a significant role in promoting development, aging, and human diseases.Among them, Huadazhi's DNBSEQTM platform technology provides high-quality tool support for research.Regarding its principle advantages, previous articles have shown that DNBSEQTM has obvious advantages in SNP targeting for single cells.
The development of single-cell sequencing technology has been advancing with each passing day, which has led to breakthroughs in many fields. The newly released “Single Cell Laboratory that can be loaded into bags”, DNBelab C series products, also provides portable, instant, and single-cell library preparation. One-stop tool support.With the MGI sequencing platform and single cell analysis software, the whole process of single cell omics research can be completed.
Research summary

Early post-implantation development of primate embryos is a milestone in mammalian development, but this stage is also one of the least clear periods of embryo development research.In this study, the cynomolgus embryos were successfully cultured for 20 days in vitro, and the in vitro cultured embryos were observed 9 to 20 days after fertilization. It was found that the in vitro cultured embryos showed high morphological and gene expression characteristics consistent with the in vivo developmental embryos.
In this paper, single-cell sequencing technology was used to sequence embryonic cells cultured at 7 time points in vitro, and the differentiation and gene expression trajectories similar to those in vivo were found.For the first time, the study utilized an in vitro system to analyze important molecular and cellular biological events after primate embryo implantation, especially during the primary bowel movement.The results of the study will help to deepen the understanding of early embryo development after implantation, and also have guiding significance for cell replacement therapy and organ regeneration research.
Research methods
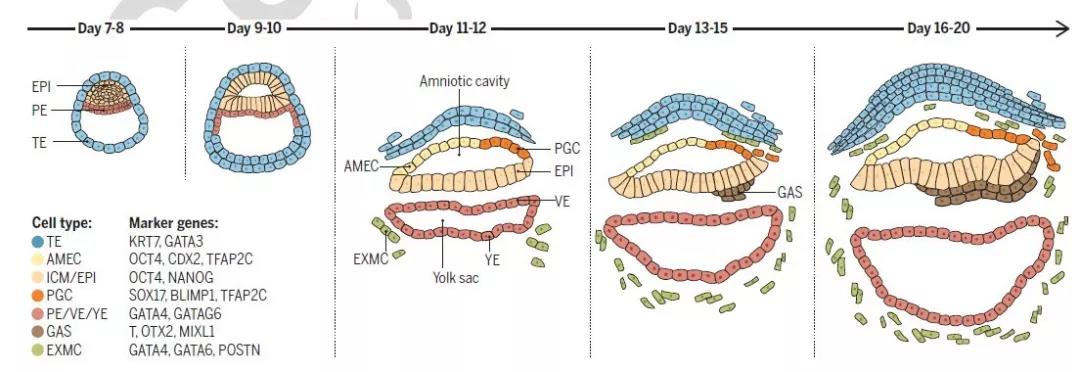
Changes in primate fertilized eggs from day 7 to 20
(Remarks: epi: ectoderm pe: primitive endoderm te: trophoblast amec: amnion epithelial cells pgc: primordial germ cells exmc: extramedullary mesenchymal cells gas: gastrort cells)
Partial results display
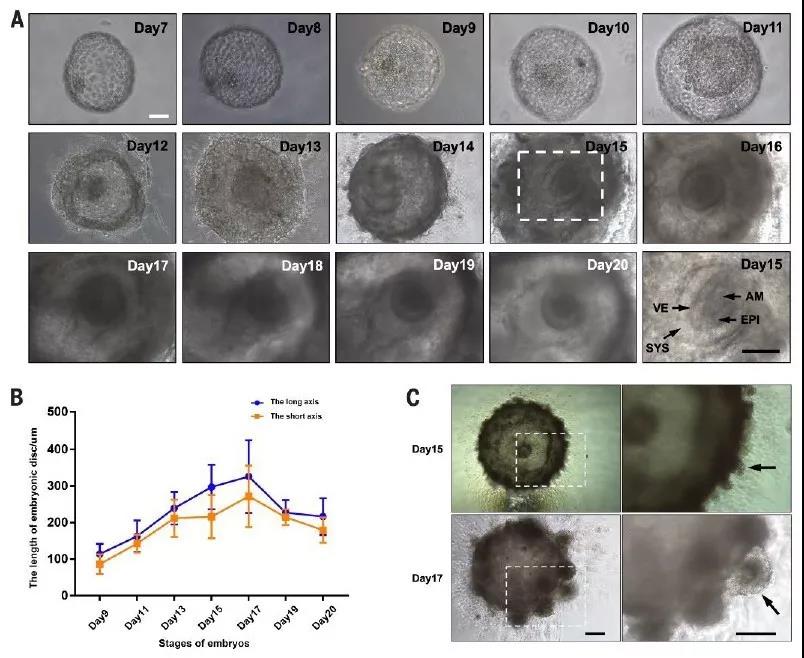
In this study, the in vitro culture system of cynomolgus monkey embryos was optimized so that it could be cultured for 20 days in vitro. It can be observed in Figure 1 that the cell mass (icm) cells proliferated to form a disc-like structure within 20 days.
Figure 1 In vitro culture system of cynomolgus monkey embryos (a: embryo shape observed on days 7-20; b: length of embryonic disc; c: structure of villus samples of in vitro cultured embryos on days 15 and 17; arrows indicate villous structures )
The immunohistochemical method was used to analyze the protein expression of embryos cultured in vitro at different stages. The cultured embryos showed high morphological and gene expression characteristics consistent with the developmental embryos in vivo (Fig. 2, Fig. 3). Starting from the 14th day, The presence of t+/sox17+/tfap2c+ cells in the amnion (Fig. 2) is consistent with the expression of embryos in vivo (Fig. 3), revealing that the amniotic epithelium is the potential origin of primordial germ cells.
Figure 2 Immunofluorescence staining results (on days 13, 14, 16 and 17 of in vitro cultured embryos, arrows indicate primordial germ cells in the amniotic membrane; short arrows indicate primordial germ cells below the ectoderm)
Figure 3 shows the expression of primordial germ cell marker gene
Single cell transcriptome and chromatin accessibility analysis
In this paper, single-cell sequencing technology was used to sequence embryonic cells cultured at 7 time points in vitro (9th, 11th, 13th, 15th, 17th, 19th and 20th days), and t-SNE dimensionality analysis was performed. Groups of cells (distributed from ectoderm (EPI), primitive endoderm (PE, VE/YE), trophectoderm (TE), and extraembryonic mesenchyme (EXMC), these cells are identical to the cell types identified in vivo.
Figure c, d below is the trophoblastic ectoderm (te) differentiation trajectory: te cells gradually differentiate from day 11 and with the decrease of cdx2 expression, te cells are divided into two types, and cells expressing high levels of tceal4 may be nourished. Layer cells (cts), cells expressing high levels of gcm1 may be syncytiotrophoblast cells (sts). This result confirms that the expression of the recognized trophoblast stem cell marker cdx2 in mice is not maintained in primate early embryo development.
Figure E below is the developmental trajectory of primitive endoderm (PE, VE/YE) cells, which can differentiate into two cell clusters.Cell cluster 1 and cell cluster 2 express high levels of apoa2 and cxcr4, respectively.
Figure f below shows the expression of cell cluster 1 and lipid metabolism and transport, while cell cluster 2 mainly expresses genes that mediate transcription and protein synthesis.
Figure G below is the differentiation trajectory of ectodermal EPI cell clusters. EPI-A and EPI-B are clustered together with early EPI cells in vivo. EPI-C is clustered with late EPI in vivo, and Gast is clustered with progaoblasts in vivo.
Discussion
Early embryonic development of primates is highly similar to humans, but studies on post-implantation embryo development in primates are still difficult to carry out, and human and material costs are enormous.
In order to further explore the molecular mechanism during the development of primate embryos after implantation, this study achieved the brood culture of cynomolgus monkeys for 20 days in vitro. The cultured embryos showed high morphological and gene expression characteristics consistent with the developmental embryos in vivo. However, the structure of the in vitro cultured embryo eventually collapsed in about 20 days, and further improvement of the culture parameters was required to achieve longer in vitro embryo development.
In vitro cultured cynomolgus embryonic cells were subjected to single-cell analysis of trophectoderm, pluripotent ectoderm, and primitive endoderm transcriptome profiles, and cell subtypes and status changes of ectodermal (epiblast) cells in differentiation were revealed and revealed. The role of the oxidative phosphorylation pathway in na?ve pluripotency maintenance; a high degree of similarity at the transcriptome level between in vitro differentiated primordial germ cell-like cells and in vivo primordial germ cells was confirmed.Through the analysis of cell ligand and receptor gene expression, the interaction between ectoderm, pluripotent ectoderm, and primitive endoderm lineage cells is revealed, such as the important role of FGF and WNT pathway in lineage differentiation.
In vitro culture of embryos has opened up a new research platform that will help to understand the dynamic molecular and cellular changes during the early development of primates, combined with cell tracking technology and single-cell sequencing technology, will help to determine cell development and differentiation. The rules and pathways.For the first time, the research work uses in vitro systems to reveal key developmental events and complex molecular mechanisms of early non-human primate embryogenesis after implantation, which contribute to the understanding of post-implantation embryo development in humans.


 Sequencer Products: SEQ ALL
Sequencer Products: SEQ ALL











 Technologies
Technologies Applications
Applications Online Resources
Online Resources Data Bulletins
Data Bulletins Service & Support
Service & Support Global Programs
Global Programs Introduction
Introduction Newsroom
Newsroom Doing Business With Us
Doing Business With Us Creative Club
Creative Club













