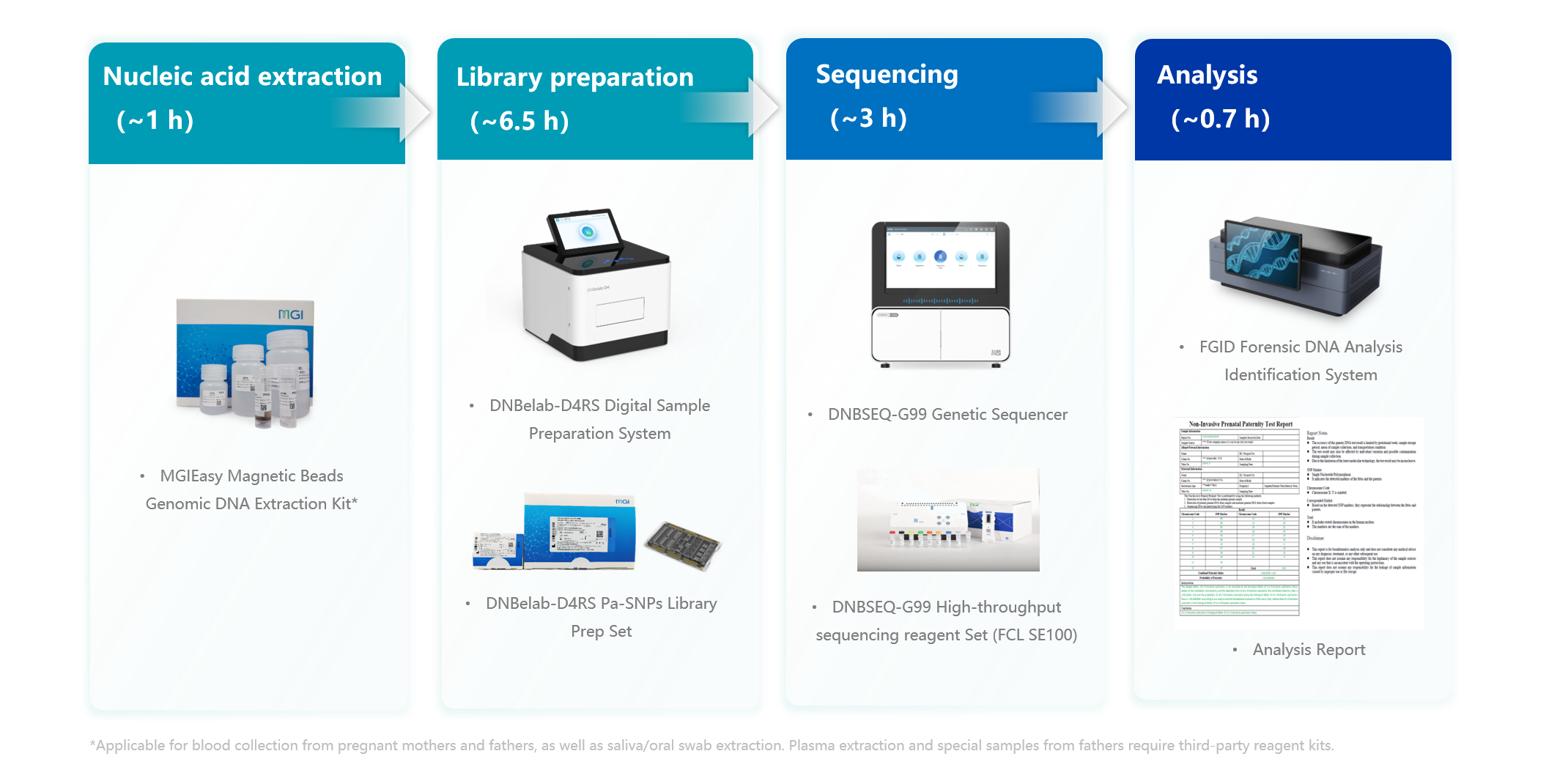
Non-invasive Prenatal Paternity Testing Package
As technology advances, the need for non-invasive prenatal paternity testing is growing. Traditional prenatal paternity testing methods are limited by the high sample input (typically demanding ~150 ng of gDNA and 10 ng-60 ng of cDNA), which not only increases the difficulty of sample collection but also poses significant challenges to subsequent experimental processes. The complex procedural steps, lengthy experimental cycles, and the reliance on high-standard laboratory environmental control and skilled personnel make traditional methods costly and highly susceptible to laboratory contamination, thereby affecting the accuracy and reliability of the test results.
Facing these challenges, MGI has introduced a non-invasive prenatal paternity testing product package. This comprehensive product package integrates all necessary reagents and equipment for the entire process, from sample extraction and library preparation to high-throughput sequencing and data analysis, offering a more convenient, safe, and efficient full-process solution for prenatal paternity testing applications.



 Sequencer Products: SEQ ALL
Sequencer Products: SEQ ALL















 Technologies
Technologies Applications
Applications Online Resources
Online Resources Data Bulletins
Data Bulletins Service & Support
Service & Support Global Programs
Global Programs Introduction
Introduction Newsroom
Newsroom Doing Business With Us
Doing Business With Us Creative Club
Creative Club