To handle the epidemic situation, the new type of coronavirus nucleic acid test is the key, which is an important basis for clinical diagnosis and patient rehabilitation and discharge, and also a basis for judging the removal of suspected cases and the release of contacts.The research and development and production of equipment have received great attention from leaders of the Party Central Committee, the State Council and governments at all levels.
On February 28, Li Keqiang, member of the Standing Committee of the Political Bureau of the Communist Party of China Central Committee, Premier of the State Council, and leader of the Central Leading Group for New Coronavirus Pneumonia Epidemic Work, inspected the New Coronavirus detection related products of MGI and other institutions at the National New Coronavirus Pneumonia Drug Medical Device Emergency Platform.

Premier Li Keqiang inspects MGI Coronavirus detection related products
On the same day, Ying Yong, the secretary of the Hubei Provincial Party Committee and the commander of the Hubei Provincial Coronavirus Pneumonia Epidemic Prevention and Control Command, came to Wuhan MGI to investigate and inspect the production of medical supplies.


Li Keqiang visits coronavirus detection related products
According to the news webcast on February 28, Li Keqiang, member of the Standing Committee of the Political Bureau of the Communist Party of China Central Committee, Premier of the State Council, and leader of the Central Leading Group's Working Group on Response to the Coronavirus Pneumonia Epidemic, went to the National Coronavirus Pneumonia Drug Medical Device Emergency Platform Inspection. Platforms includes MGI's new coronavirus detection kit and MGI's ultra-high-throughput sequencer DNBSEQ-T7.
Premier Li Keqiang said that testing reagents have played a very important role in the prevention and control of this epidemic. The next step is to quickly develop reagents with shorter testing time, more accurate results and simpler operations. At the same time, relevant equipment and personnel training must be done. It is needed to enhance detection capabilities and better early detection, which is conducive to early isolation and early treatment, curb the spread of the epidemic, and improve the cure rate.

Ultra-high-throughput sequencer DNBSEQ-T7
MGI 2019-nCoV nucleic acid detection kit and analysis software
Under the guiding principle of "early intervention, follow-up review, and scientific approval", the State Food and Drug Administration quickly launched the emergency approval process for products tested for the coronavirus pneumonia epidemic, which only took 4 days, and was approved on January 26, 2020. 4 coronavirus pneumonia epidemic detection products from 4 companies including MGI are inspected.
Ying Yong went to Wuhan to investigate and inspect the production of medical supplies
On the morning of February 28, Ying Yong, the secretary of the Hubei Provincial Party Committee and the commander of the Hubei Provincial Coronavirus Pneumonia Epidemic Prevention and Control Headquarters, came to Wuhan MGI to investigate and inspect the production of medical supplies.
After a detailed understanding of kit production and laboratory testing, Ying Yong said, "Nucleic acid testing is an important foundation for implementing scientific prevention and treatment requirements and achieving early detection and early treatment. I hope that enterprises strictly implement various prevention and control measures, continue to increase research and development efforts, and make greater contributions to epidemic prevention and control. "
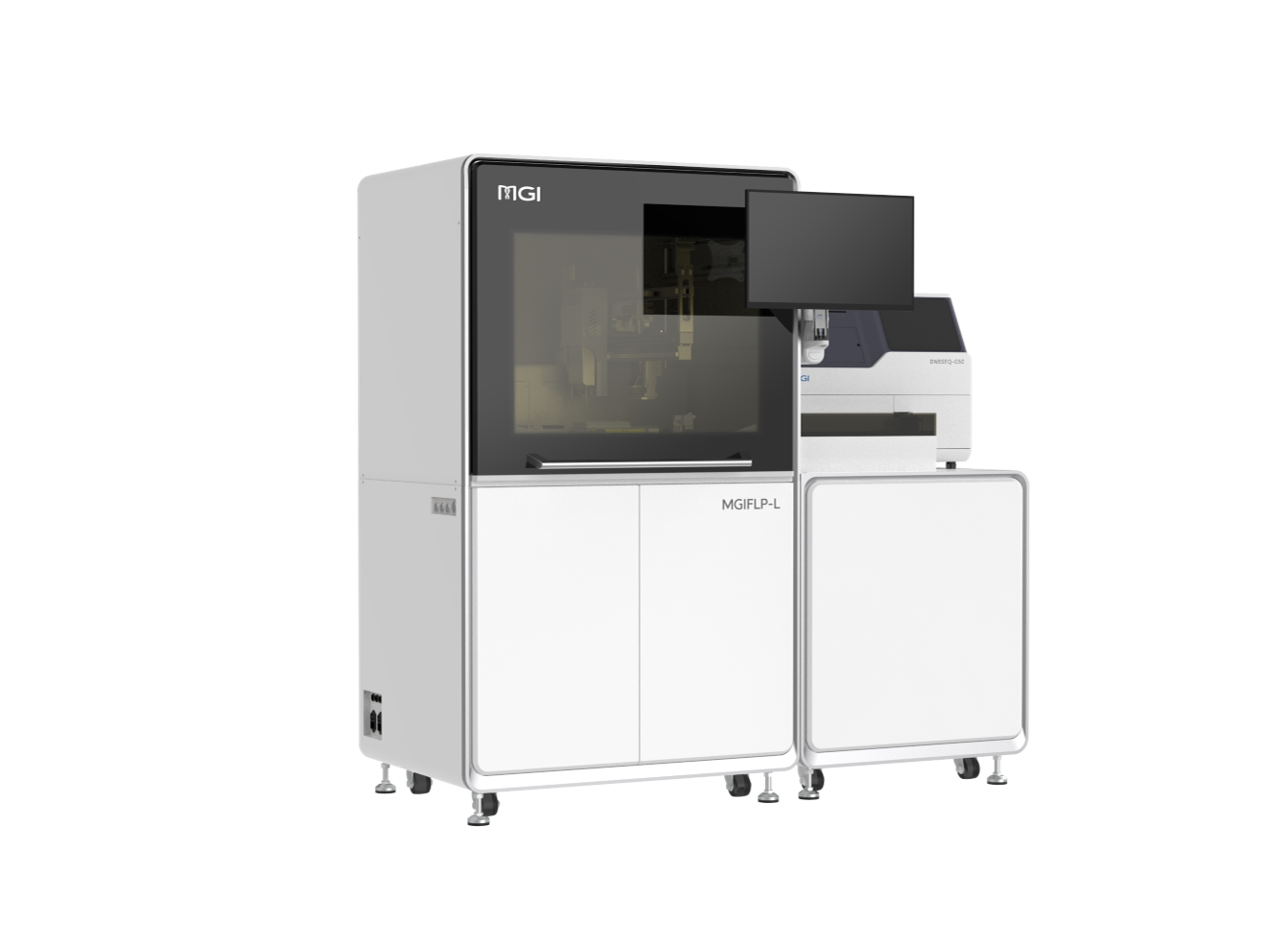
After the outbreak, MGI rapidly increased the production capacity of nucleic acid detection kits. Currently, it has produced more than 1.2 million copies of kits, and built a high-throughput sequencing laboratory (Wuhan "Huo-Yan" laboratory). The MGISP-100 and MGISP-960 automated sample preparation systems perform nucleic acid extraction, testing more than 79,000 samples in Hubei Province, effectively alleviating the shortage of kit supplies and insufficient detection capabilities.
The "Huo-Yan" model is being replicated across the country, providing scientific evidence for the diagnosis of fever patients, the screening of high-risk groups, the identification of suspected cases, the isolation of positive infections, and the screening of healthy people, etc., to combat the epidemic and restore orderly operation of society and economy Provide scientific assurance.
Wuhan Fire-Eye Lab overall improves the speed of detection of large-scale samples
In addition to equipping Wuhan's "Huo-Yan" laboratory to ensure the detection capacity of 10,000 people per day, related products made by MGI, such as high-throughput automated sample preparation systems, have assisted medical institutions and disease control in more than 30 cities and regions across the country. center.
As of February 28, MGI has received more than 300,000 samples in the country, and the daily throughput of the country has reached 50,000, which can be increased to 80,000 copies per day as needed.
Wang Jian, chairman of MGI, said that MGI always adheres to the goal-oriented, always upholds a rigorous scientific spirit, always focuses on genetic technology, and has established an independent supporting tool and a large platform system, from international cooperation to independence, to leading development and development. The large-scale engineering practice that conforms to the national conditions, innovative institutional mechanisms, complementary talent teams, and a broad vision of the world, learn and grow in the battle, correct and correct errors. These unique paradigms of MGI will continue to serve Wuhan, Hubei, and even the whole country. International prevention and control work will provide strong scientific support and guarantee.


 Sequencer Products: SEQ ALL
Sequencer Products: SEQ ALL










 Technologies
Technologies Applications
Applications Online Resources
Online Resources Data Bulletins
Data Bulletins Service & Support
Service & Support Introduction
Introduction Newsroom
Newsroom Doing Business With Us
Doing Business With Us Creative Club
Creative Club